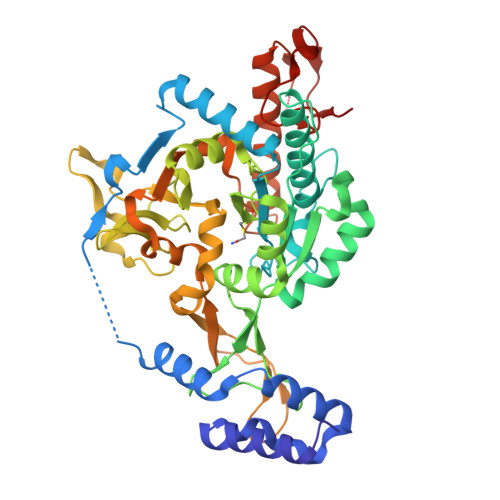
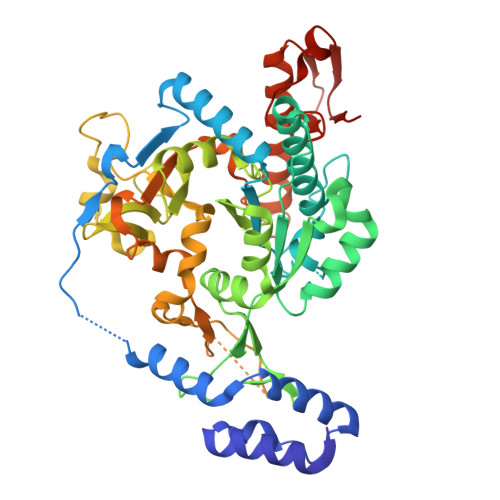
Exploration of starting points for the chemical validation of UDP-N-acetylglucosamine pyrophosphorylase in Aspergillus fumigatus
Yan, K., Stanley, M., Raimi, O., Kowalski, B., Gurvic, D., Grillenberger, S., Chen, X., Ferenbach, A.T., Dorfmueller, H., van Aalten, D.M.F.To be published.