Structural mechanisms of allosteric network regulation in the human cis-prenyltransferase complex
Giladi, M., Haitin, Y.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Dehydrodolichyl diphosphate synthase complex subunit DHDDS | 340 | Homo sapiens | Mutation(s): 0 Gene Names: DHDDS, HDS EC: 2.5.1.87 | 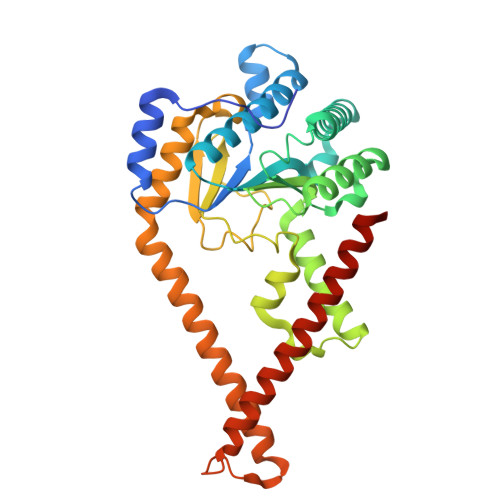 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q86SQ9 (Homo sapiens) Explore Q86SQ9 Go to UniProtKB: Q86SQ9 | |||||
PHAROS: Q86SQ9 GTEx: ENSG00000117682 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q86SQ9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Dehydrodolichyl diphosphate synthase complex subunit NUS1 | 222 | Homo sapiens | Mutation(s): 1 Gene Names: NUS1, C6orf68, NGBR EC: 2.5.1.87 |  | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96E22 (Homo sapiens) Explore Q96E22 Go to UniProtKB: Q96E22 | |||||
PHAROS: Q96E22 GTEx: ENSG00000153989 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96E22 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FPS (Subject of Investigation/LOI) Query on FPS | D [auth A] | S-[(2E,6E)-3,7,11-TRIMETHYLDODECA-2,6,10-TRIENYL] TRIHYDROGEN THIODIPHOSPHATE C15 H28 O6 P2 S MYMLCRQRXFRQGP-YFVJMOTDSA-N |  | ||
| IPE (Subject of Investigation/LOI) Query on IPE | C [auth A] | 3-METHYLBUT-3-ENYL TRIHYDROGEN DIPHOSPHATE C5 H12 O7 P2 NUHSROFQTUXZQQ-UHFFFAOYSA-N |  | ||
| MG (Subject of Investigation/LOI) Query on MG | E [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 184.399 | α = 90 |
| b = 184.399 | β = 90 |
| c = 112.575 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| XDS | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Israel Science Foundation | Israel | 1653/21 |
| United States - Israel Binational Science Foundation (BSF) | United States | 2023190 |
| Other private | Israel Cancer Research Fund 1289067 |