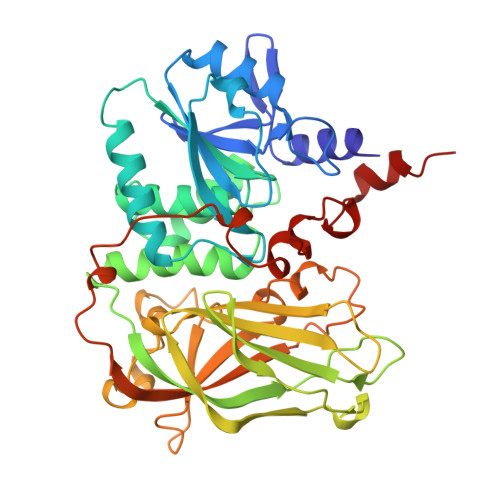
Elucidating PTEN conformational dynamics and phosphatase regulation via integrative modeling and mutation prediction
Dawson, J.E., Smith, I.N., Tushar, A.M., Eng, C.(2025) Structure
- PubMed: 40614725
- DOI: https://doi.org/10.1016/j.str.2025.06.002
- Primary Citation of Related Structures:
9A90, 9A91, 9A92, 9A93, 9A94, 9A95, 9A96, 9A97, 9A98, 9A99, 9A9A, 9A9B, 9A9C, 9A9D, 9A9E, 9A9F - PubMed Abstract:
PTEN (Phosphatase and TENsin homolog deleted on chromosome ten) is a major tumor suppressor gene that is frequently mutated or lost under cancerous conditions. PTEN is a dual-specificity phosphatase that negatively regulates the PI3K/AKT/mTOR signaling pathway at the plasma membrane (PM). Its functional regulation and cellular localization are known to be conformationally driven. Access to the PM is phosphoregulated by open and closed PTEN forms. However, clarifying the underlying structural mechanisms is still an open avenue of research. Here, we apply an integrative structural modeling approach, combining coarse-grained and all-atom molecular dynamics with experimental crosslinking mass spectrometry. Conformational exchange between an "eased" form and a "strained" form brings the protein's phosphatase and C2 domains closer together, blocking the catalytic site, and affecting the loops involved in PM binding. Our full-length PTEN models, AlphaMissense, and RaSP were used to better predict the consequences of PTEN mutations.
Organizational Affiliation:
Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, NE-50, Cleveland, OH 44195, USA. Electronic address: dawsonj8@ccf.org.